Figures reveal early pandemic rush for covid-related trademarks

Cape Verde, an island state formed by an archipelago of 10 islands, benefits from its geographic position and has an economy based on the services sector, including trade, transport, tourism, public services, and exports of fish and clothing.
Despite this, natural resources are scarce, with prolonged water shortages enhanced by long and cyclical periods of drought, and low-fertility soils on several of the islands. Although about 40% of the population lives in rural areas, food production makes up a low percentage of GDP (4.9% in 2020), with approximately 70% of food imported. Cape Verde imports fuel and machinery, as it produces neither.
Given these facts it is unsurprising that overseas companies have filed trademark applications under Nice classes related to scarce goods: Class 12 (vehicles); Class 4 (lubricants and fuel); and Classes 29 and 30 (meats and processed foods; staple foods).
In the early months of 2020, medical and pharmaceutical industries focused on protecting their IP rights in several countries to safeguard the ownership of their intellectual and industrial creations, especially those involving the fight against covid-19. Cape Verde was no exception and it’s now clear that the start of 2020 was marked by an increase in trademark applications under Nice Classes 1 (chemicals) and 5 (pharmaceuticals and medical supplies)
While these pharmaceutical and health-related products and services are form only a small percentage of the trademark rights in existence in Cape Verde, it is a significant group.
Comprising only trademark applications published in the IP Bulletins (we exclude trademark applications awaiting publication, as these have not been made known to the public yet), the following are worth highlighting.
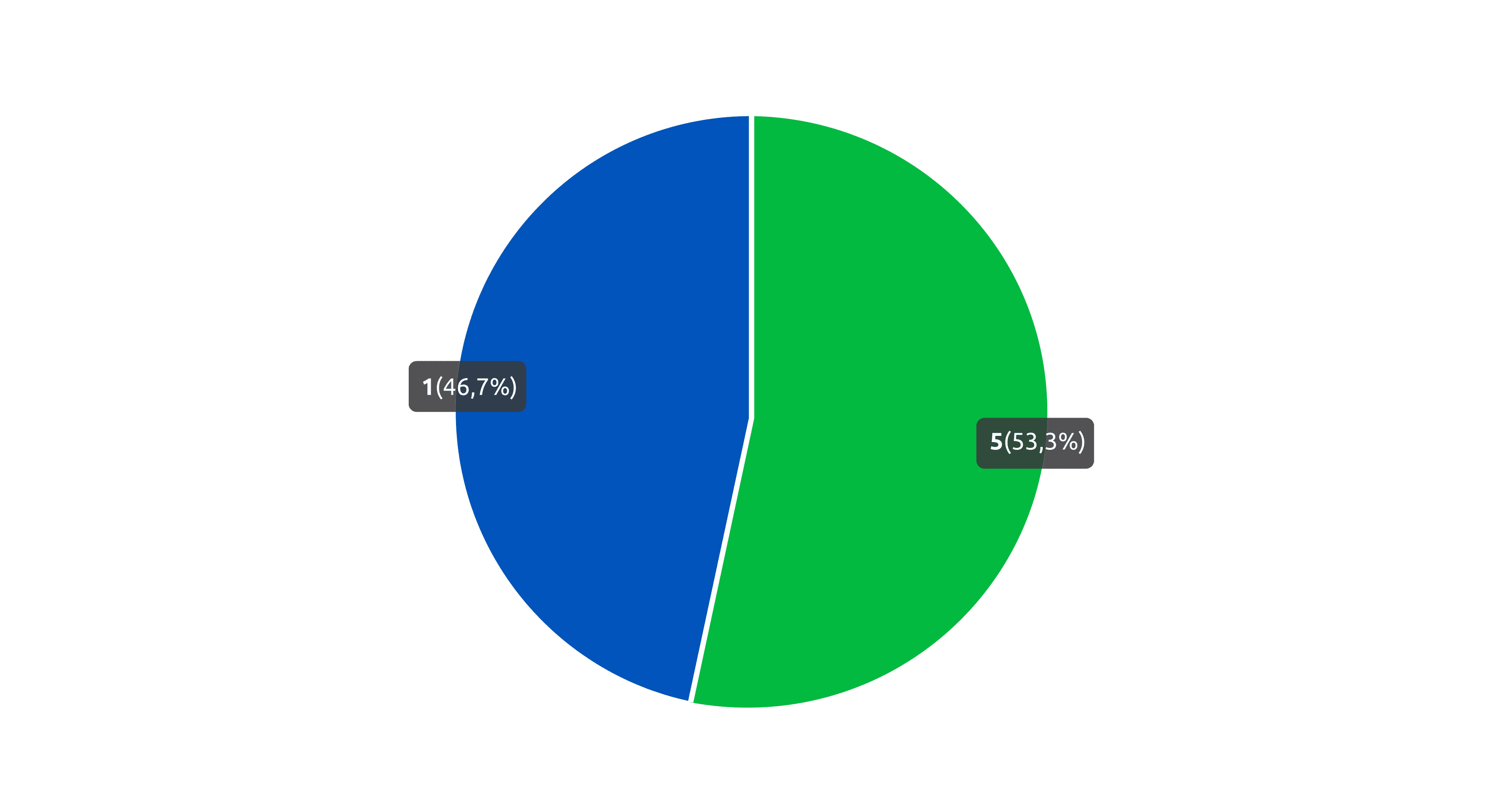
There are 13 trademarks under Classes 1 and 5 published from 2020 until today. Of this total, 53.3% were applied for in Class 5 and 46.7% were applied for in Class 1.

The top three proprietors were:
- AstraZeneca UK Limited;
- Abbott Rapid Diagnostics International Subsidiary Unlimited Company; and
- Abbott Rapid Diagnostics International Unlimited Company.
Out of these, AstraZeneca UK Limited, focused on Class 5, namely on products pharmaceutical preparations and substances, aiming more directly for vaccination. On the other hand, Abbott seeks protection on both Classes 1 and 5, aiming for vaccination, but also for diagnostic preparations used in science.
The top product categories were:
- pharmaceuticals;
- diagnostic preparations used in science; and
- Drug testing reagents.
Besides this focus on Classes 1 and 5, there has also been a significant uptick in covid-related marks in other industries with products and services that aid in the fight against coronavirus. For instance, the transportation industry (with services of urgent delivery of drugs and vaccines), the clothing industry (with the necessary wearable materials to protect health professionals and the population) and the machinery industry (with products and services orientated for the medical equipment), among many others.
This is a co-published article, which was originally published in the World Intellectual Property Review (WIPR).





