
Patents referred to medicaments in the context of the ARIPO and its Contracting States
The African Regional Intellectual Property Organization (ARIPO) is empowered to grant and administer patents in accordance with the provisions of the Harare Protocol on Patents and Industrial Designs. According to Harare Protocol, patents shall be granted for any inventions, in all fields of technology, provided that they meet the patentability requirements: novelty, inventive step and industrial application.
On the other hand, concerning medicaments patents, there are some deviations between the legal background established in the Harare Protocol and the National Laws from some Contracting States.
This article will focus on some aspects related to medicaments patents granted by ARIPO in the context of national laws of some Contracting States which do not allow patents in this field of technology or have very restrictive requisites to grant a patent on this subject matter.
Discussion and some results of medicaments patents granted by ARIPO
Nowadays ARIPO has the following 19 Contracting States: Botswana, The Gambia, Ghana, Kenya, Lesotho, Liberia, Malawi, Mozambique, Namibia, Rwanda, Sao Tome and Principe, Sierra Leone, Somalia, Sudan, Swaziland, Uganda, United Republic of Tanzania, Zambia and Zimbabwe.
The patents granted by ARIPO shall, in each of the Contracting States for which it is granted, have the effect of and be subject to the same conditions as a national patent granted by that State.
The prosecution in ARIPO begins with the patent application, which must contain one or more designations of Contracting States. If ARIPO decides to grant a patent, it shall notify the applicant and each designated State. A copy of the search and examination report shall be attached to the said notification. The designated State shall have 6 months within which to respond to the notification. This deadline is very relevant in the cases wherein medicaments patents are involved, because a designated State has an opportunity to clearly state that the patent shall not be valid in its territory, per instance, due a prohibition of its National Law.
After expiration of the said 6 months, ARIPO shall grant and publish the patent, which shall have effect in those designated States which have not issued any communication preventing that patent from producing effect in its territory, because of the nature of the invention, a patent cannot be registered or granted or has no effect under the national law of that State.
 Some Contracting States have the status of Least-developed country (LDC) members of the World Trade Organization (WTO) and are allowed to maintain maximum flexibility in their approach to patenting pharmaceutical products until at least 2033. Therefore, Liberia and Uganda establish in their respective National Laws that pharmaceutical products shall not be regarded as inventions and shall be excluded from patent protection until 1st January 2016 or such other period as may be granted to Uganda or least developed countries by the Council responsible for administering the Agreement on trade related aspects of intellectual property under the WTO.
Some Contracting States have the status of Least-developed country (LDC) members of the World Trade Organization (WTO) and are allowed to maintain maximum flexibility in their approach to patenting pharmaceutical products until at least 2033. Therefore, Liberia and Uganda establish in their respective National Laws that pharmaceutical products shall not be regarded as inventions and shall be excluded from patent protection until 1st January 2016 or such other period as may be granted to Uganda or least developed countries by the Council responsible for administering the Agreement on trade related aspects of intellectual property under the WTO.
On the other hand, Gambia, Lesotho, Mozambique, Sierra Leone and United Republic of Tanzania are members of WTO and are recognized as LDC and do not have exclusions to medicaments patents in their respective Laws. Sao Tome and Principe, Somalia and Sudan are least-developed countries and are negotiating to join the WTO and, currently, also do not have exclusions to medicaments patents in their National Laws.
In the case of Liberia, even using its current status of LDC to postpone the granting of medicaments patents, the National Law has already provisioned some restrictive and precise requirements “in the case of chemical substances of therapeutic use, salts, esters, ethers, polymorphs, metabolites, pure form, particle size, isomers, mixtures of isomers, complexes, combinations and other derivatives”. Indeed, it is established that “advantageous properties such as thermodynamic stability, lower hygroscopicity and potency of a substance shall not be deemed to constitute an enhancement of therapeutic efficacy”.
Malawi, Namibia, Rwanda, Zambia and Zimbabwe have some restrictions in their National Laws concerning the pharmaceutical technological field.
The Patents Acts of Malawi and of Zimbabwe state that a patent application may be refused if “it claims as an invention a substance capable of being used as food or medicine which is a mixture of known ingredients possessing only the aggregate of the known properties of the ingredients” or if “it claims as an invention a process producing such a substance by mere admixture”.
The Industrial Property Act of Namibia states that “new uses, methods of use, forms, properties of a known product or substance and already used for specific purposes and changes of shape, dimensions, proportions or materials in the subject matter applied for, except where the qualities of the subject matter are essentially altered or where its use solves a technical problem that did not previously have an equivalent solution” are excluded from patentability. This provision is especially relevant to “salts, esters, ethers, polymorphs, metabolites, pure form, particle size, isomers, mixtures of isomers, enantiomers, complexes, combinations, compositions, formulations, dosage forms, admixtures and other derivatives of known substances”.
The Law on the Protection of Intellectual Property of Rwanda has some diverse requirements, wherein in the Law is stated that “methods for treatment of the human or animal body by surgery or therapy, as well as diagnostic methods practiced on the human or animal body are excluded from patent protection; nevertheless, this provision shall not apply to products for use in any of those methods”. Furthermore, known substances for which a new use has been discovered are excluded from patent protection; but this provision shall not apply to the use itself, where it can constitute an invention. However, it is also stated that “pharmaceutical products, for the purposes of international conventions to which Rwanda is party” are also excluded from patent protection.
The Patents Act of Zambia clearly states that a patent shall not be granted for “new uses of a known product, including the second use of a medicine”.
In order to identify some influences of the differences among the Harare Protocol and the National Patent Laws of the Contracting States, we have performed a search in ARIPO patents database. First, we have identified the number of patent applications in ARIPO with filing date in the range from 2008 to 2018 and we have done the same search for each one of the Designated States. Then, within the same sample, we have searched the total of medicaments patents, which were classified with at least one of the following International Patent Classification (IPC): A61K9, A61K31, A61K33, A61K35, A61K36, A61K38, A61K39, A61K41, A61K45, A61K47 or A61K48. Finally, we have performed the search for the granted patents in these late results. The results can be viewed in the table 1, wherein the States that have restriction about the granting of medicaments patents are highlighted.
Table 1: landscape of medicaments patents in ARIPO
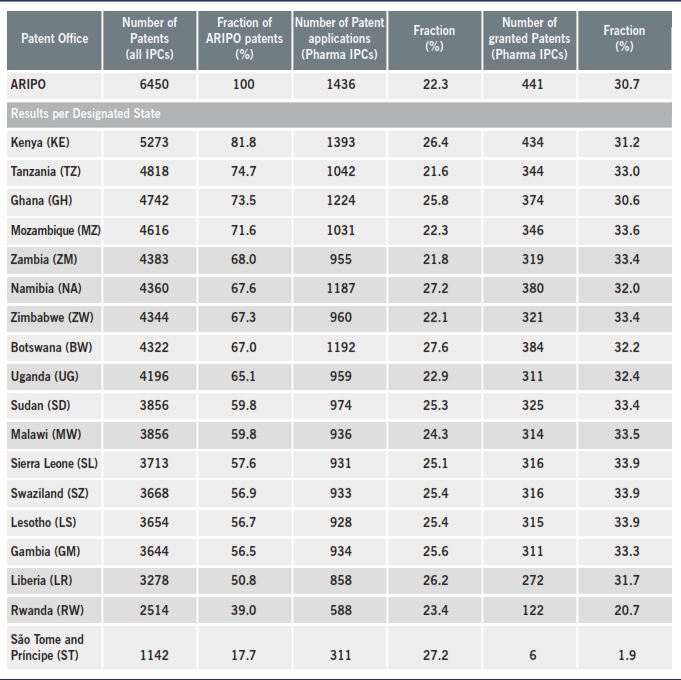
The results in table 1 show that about 25% of the patent applications in ARIPO belong to pharmaceutical technological field, wherein this share is very similar amongst the Designated States. We have searched for the current status of the medicaments patent applications in the ARIPO database. With the exceptions of Rwanda and Sao Tome and Principe, the granting rate is about 33% for the other countries. It is remarkable that amongst the overall decisions, the refusals fraction is very low, as presented in table 2.
Table 2: status of medicaments patents in ARIPO
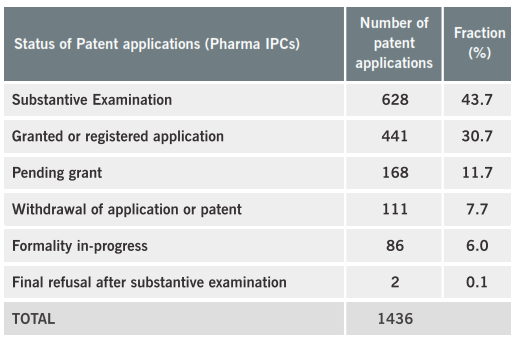
Unfortunately, some important information about the status of granted patents are not available online in ARIPO website, as the scope of the claims after granting or the updated status if some Contracting State has communicated the objection to a medicament patent granted.
Conclusions
Despite of having a significant share of the overall number of patent applications in ARIPO, the status of medicaments patents in some ARIPO’s Designated States is quite uncertain, due the provisions in their respective National Laws referred to restrictions on medicaments patentability, which provisions are absent on Harare Protocol. Besides, there is lack of information online in ARIPO and in the National Offices websites about the current status of the medicaments patents.
Lista de Territórios
Não existem resultados para a sua pesquisa.
- África
- África do Sul
- Angola
- Argélia
- Benin
- Botsuana
- Burkina Faso
- Burundi
- Cabo Verde
- Camarões
- Chade
- Comores
- Costa do Marfim
- Djibuti
- Egito
- Eritreia
- Eswatini (Suazilândia)
- Etiópia
- Gabão
- Gâmbia
- Gana
- Guiné
- Guiné-Bissau
- Guiné-Equatorial
- Lesoto
- Libéria
- Libia
- Madagáscar
- Maiote
- Malaui
- Máli
- Marrocos
- Maurícias
- Mauritânia
- Moçambique
- Namíbia
- Níger
- Nigéria
- Quénia
- República Centro-Africana
- República Democrática do Congo
- República do Congo
- Reunião
- Ruanda
- Saara Ocidental
- São Tomé e Principe
- Seicheles
- Senegal
- Serra Leoa
- Somália
- Sudão
- Sudão do Sul
- Tanzânia
- Togo
- Tunísia
- Uganda
- Zâmbia
- Zanzibar
- Zimbábue
- África (OAPI)
- África (ARIPO)
- Mais Territórios
- Macau
- Maldivas
- Portugal
- Timor Leste
- Marca da União Europeia (EUIPO)
- Marca Internacional (Sistema de Madrid)
- Patente Europeia (IEP)
- Tratado de Cooperação em matéria de Patentes (PCT)




